Common ion effect on solubility pogil answers explores the fascinating interplay between solubility equilibrium and the presence of common ions. This concept finds wide-ranging applications in chemistry, analytical chemistry, and beyond. By delving into the intricacies of this phenomenon, we gain a deeper understanding of the factors that govern the solubility of ionic compounds.
Solubility equilibrium, a dynamic state where the rate of dissolution equals the rate of precipitation, plays a pivotal role in the common ion effect. The presence of a common ion shifts this equilibrium towards the formation of the solid phase, decreasing the solubility of the ionic compound.
Le Chatelier’s principle provides a valuable tool for predicting the effects of common ion effect, guiding us in understanding how changes in concentration affect the equilibrium position.
Common Ion Effect
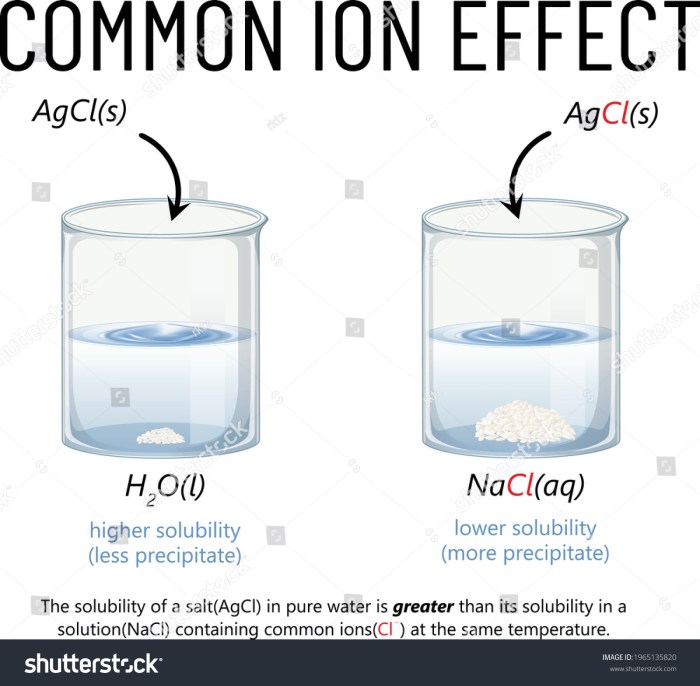
The common ion effect is the suppression of the ionization of a weak electrolyte when a strong electrolyte containing a common ion is added to the solution. The common ion is an ion that is present in both the weak and strong electrolytes.
For example, if we add sodium chloride (NaCl) to a solution of acetic acid (CH 3COOH), the ionization of acetic acid is suppressed. This is because the sodium ions (Na +) from the NaCl combine with the acetate ions (CH 3COO –) from the acetic acid to form sodium acetate (CH 3COONa), which is a strong electrolyte.
The formation of sodium acetate reduces the concentration of acetate ions in the solution, which in turn reduces the ionization of acetic acid.
Applications of Common Ion Effect in Chemistry
The common ion effect has a number of applications in chemistry, including:
- The precipitation of ions from solution
- The separation of ions from solution
- The control of the pH of a solution
Solubility Equilibrium
Solubility equilibrium is the state of a solution in which the rate of dissolution of a solute is equal to the rate of precipitation of the solute. At solubility equilibrium, the concentration of the solute in the solution is constant.
The common ion effect can affect solubility equilibrium by shifting the equilibrium to the side of precipitation. This is because the addition of a common ion to the solution reduces the concentration of the solute in the solution, which in turn reduces the rate of dissolution and increases the rate of precipitation.
Mathematical Equations to Represent Solubility Equilibrium
The solubility equilibrium constant (K sp) is a constant that is used to represent the solubility of a solute in a solution. The K spis equal to the product of the concentrations of the ions of the solute in the solution.
For example, the K spfor calcium carbonate (CaCO 3) is:
K sp= [Ca 2+][CO 32-]
If the concentration of calcium ions in a solution is increased, the K spwill remain constant. This means that the concentration of carbonate ions in the solution must decrease in order to maintain the K sp. This decrease in the concentration of carbonate ions will shift the solubility equilibrium to the side of precipitation, causing more calcium carbonate to precipitate out of solution.
Le Chatelier’s Principle
Le Chatelier’s principle is a principle that states that if a change is made to a system at equilibrium, the system will shift in a direction that counteracts the change. This principle can be used to predict the effects of the common ion effect on solubility equilibrium.
For example, if we add a common ion to a solution of a weak electrolyte, the solubility equilibrium will shift to the side of precipitation. This is because the addition of the common ion reduces the concentration of the solute in the solution, which in turn reduces the rate of dissolution and increases the rate of precipitation.
This shift in the equilibrium is in accordance with Le Chatelier’s principle, as the system is shifting in a direction that counteracts the change that was made (the addition of the common ion).
Examples of How Le Chatelier’s Principle Can Be Used to Predict the Effects of Common Ion Effect
- If we add sodium chloride to a solution of acetic acid, the ionization of acetic acid will be suppressed. This is because the sodium ions from the sodium chloride combine with the acetate ions from the acetic acid to form sodium acetate, which is a strong electrolyte.
The formation of sodium acetate reduces the concentration of acetate ions in the solution, which in turn reduces the ionization of acetic acid.
- If we add calcium chloride to a solution of calcium carbonate, the solubility of calcium carbonate will decrease. This is because the calcium ions from the calcium chloride combine with the carbonate ions from the calcium carbonate to form more calcium carbonate.
The formation of more calcium carbonate reduces the concentration of carbonate ions in the solution, which in turn reduces the solubility of calcium carbonate.
Experimental Observations
The common ion effect can be demonstrated by a number of experiments. One simple experiment is to add sodium chloride to a solution of acetic acid and measure the pH of the solution. The pH of the solution will decrease as the ionization of acetic acid is suppressed.
Another simple experiment is to add calcium chloride to a solution of calcium carbonate and measure the amount of calcium carbonate that precipitates out of solution. The amount of calcium carbonate that precipitates out of solution will increase as the solubility of calcium carbonate decreases.
Table to Summarize the Experimental Observations, Common ion effect on solubility pogil answers
| Experiment | Observation |
|---|---|
| Add sodium chloride to a solution of acetic acid | The pH of the solution decreases |
| Add calcium chloride to a solution of calcium carbonate | The amount of calcium carbonate that precipitates out of solution increases |
Applications in Analytical Chemistry: Common Ion Effect On Solubility Pogil Answers
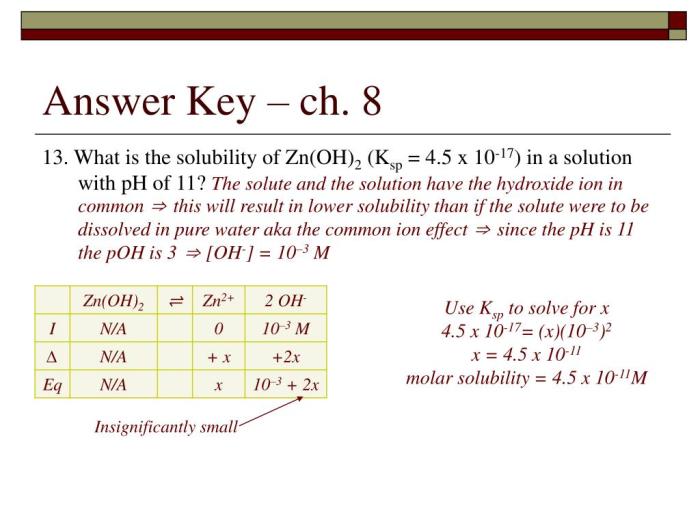
The common ion effect has a number of applications in analytical chemistry, including:
- The separation of ions from solution
- The determination of the concentration of ions in solution
- The control of the pH of a solution
Examples of Analytical Techniques That Utilize Common Ion Effect
- Ion exchange chromatography
- Ion selective electrodes
- Potentiometric titrations
FAQs
What is the common ion effect?
The common ion effect refers to the decrease in the solubility of an ionic compound when a common ion is added to the solution.
How does the common ion effect affect solubility equilibrium?
The common ion effect shifts the solubility equilibrium towards the formation of the solid phase, decreasing the solubility of the ionic compound.
What is Le Chatelier’s principle and how does it apply to the common ion effect?
Le Chatelier’s principle states that if a change is applied to a system in equilibrium, the system will shift in a direction that counteracts the change. In the context of the common ion effect, adding a common ion shifts the equilibrium towards the formation of the solid phase, decreasing the solubility.